Abstract
Aim: All-trans retinoic acid (ATRA) results in high response rates in acute promyelocytic leukemia (APL), but has not had a significant impact on non-APL forms of acute myeloid leukemia (AML), even though retinoic acid receptors have been implicated in hematopoietic maturation and self-renewal. RXRs bind as heterodimers with RARs, and are expressed at higher levels in AML cells than are RARs. We sought to determine whether natural RXR ligands exist in primary leukemia cells, whether RXR ligands might augment or inhibit leukemic cell growth, and whether RXRs might be rational targets for AML therapeutics.
Background: Retinoid receptors are nuclear hormone receptors, induced during terminal myeloid maturation with highest expressed in M4 and M5 AML. Retinoids serve as intracellular messengers and are activating ligands for the retinoic acid receptors (RAR) and retinoid X receptors (RXR).
Methods: We developed an in vivo reporter assay that measures intracellular, activating nuclear receptor ligands in mouse bone marrow cells. We generated transgenic mice with GFP regulated by a UAS promoter. The yeast Gal4 transcription factor recognizes UAS promoter sequences. When a fusion of the Gal4-DNA binding domain (DBD) and a retinoid receptor ligand-binding domain (LBD) is expressed in UAS-GFP bone marrow cells, and an activating ligand is present, the cells express GFP. Leukemia was generated in bone marrow cells from these mice and from Mx1-Cre x Rxrαfl/fl x Rxrβfl/fl mice using MSCV-MLL-AF9 retrovirus.
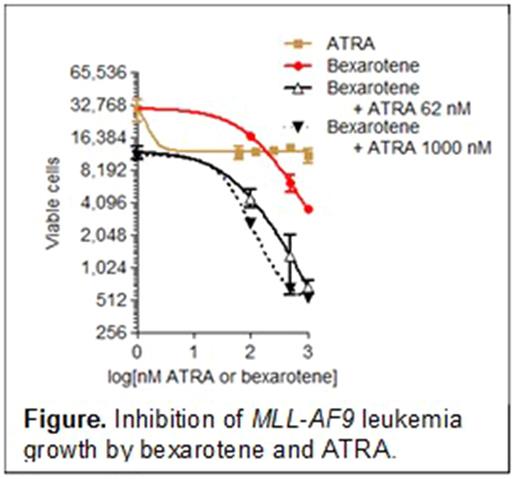
Results: We found that intermediate levels of RXRA ligands are present in mouse MLL-AF9 leukemia cells in vivo. To determine if activation of RXRA is required for MLL-AF9 cell growth, we transduced MSCV-MLL-AF9 retrovirus into bone marrow cells from Mx1-Cre+ x Rxrαfl/fl x Rxrβfl/fl mice and transplanted these into recipient mice. Mx1-Cre is "leaky." Without external activation of Mx1-Cre (i.e. without pIpC treatment) we found that all subsequent leukemias were associated with deleted ("floxed out") Rxra and Rxrb alleles, suggesting that the deleted allele was positively selected for. In secondary transplants, we found that MLL-AF9 leukemias with deleted Rxrα and Rxrβ were associated with shorter survival when compared with MLL-AF9 leukemias with wild type Rxrα and Rxrβ, suggesting that naturally occurring RXR ligands suppressed leukemic growth. To determine if pharmacologic activation of retinoid receptors could inhibit MLL-AF9 cell growth, we treated MLL-AF9 cells ex vivo with ATRA and bexarotene. We found that ATRA modestly inhibited growth, and this effect reached a plateau at 62 nM (this may partially explain the limited clinical efficacy of ATRA in non-APL AML) (see Figure). In contrast, the EC50 of bexarotene was 125 nM and the EC50 decreased to 54 nM when bexarotene was combined with 62 nM ATRA. Higher doses of ATRA decreased the bexarotene EC50 further to 43 nM and resulted in near complete culture collapse. Finally, to determine whether combination bexarotene and ATRA inhibited cell growth by inducing differentiation or apoptosis, we examined cytomorphology, cell division by Celltrace Violet staining (similar to CFSE), and Annexin V. Unexpectedly, we observed no evidence of differentiation, but rather increased Annexin V associated with ATRA and bexarotene-induced growth arrest.
Conclusion: RXRs act as tumor suppressors in MLL-AF9 murine leukemia cells in vivo, and the activation of RXR/RAR heterodimers with bexarotene and all-trans retinoic acid leads to synergistic apoptosis and growth arrest. Additional experiments are now being performed to test the efficacy of this dug combination in vivo and assess their potential use in elderly relapsed/refractory AML.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal